This page provides in-depth technical information for health practitioners and anyone wishing to have a deeper understanding of the diseases and conditions GREAT & SMALL products have been formulated to aid.
This article outlines the structure and function of joints, and the current understanding of the development of arthritis. It explains the function of chondroprotective agents which slow down the progression of disease and help restore normal joint function.
The synovial joint is composed of 2 opposing articular surfaces that are lined by cartilage which is attached to underlying trabecular bone and held in correct alignment by ligaments and capsule. The peri-articular structures are integral to joint structure and function. The joint capsule produces and contains joint fluid which lubricates the joint and is the carrier for nutrition to the cartilage. The subchondral bone protects and supports the overlying cartilage by remaining flexible and acting as a shock absorber.
This article outlines the structure and function of joints, and the current understanding of the development of arthritis. It explains the function of chondroprotective agents which slow down the progression of disease and help restore normal joint function.
The synovial joint is composed of 2 opposing articular surfaces that are lined by cartilage which is attached to underlying trabecular bone and held in correct alignment by ligaments and capsule. The peri-articular structures are integral to joint structure and function. The joint capsule produces and contains joint fluid which lubricates the joint and is the carrier for nutrition to the cartilage. The subchondral bone protects and supports the overlying cartilage by remaining flexible and acting as a shock absorber.
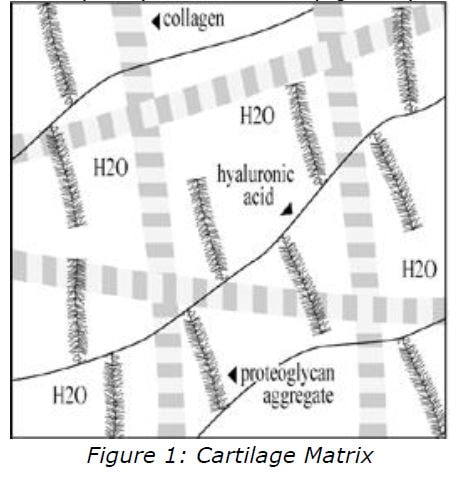
Cartilage is an avascular connective tissue which is composed of two elements:
- cellular component (5%) - the chondrocytes
- extracellular component (95%) - the matrix (figure 1).
Chondrocytes produce and maintain the matrix. The matrix in turn supplies the chondrocytes with an enviromment conducive to their continued existence in the face of a high level of mechanical stress. Water com-prises approximately 70% of the matrix, the remainder consists primarily of collagen(especially collagen II) which is meshed with proteoglycan aggregates.
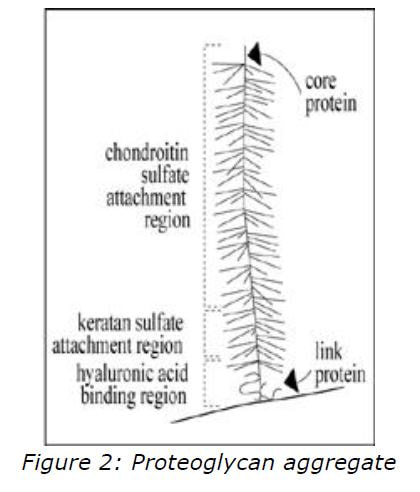
Collagen provides tensile strength and structural support; and proteoglycans provide compressive strength. The hydrophilic nature of this structure allows it to attract water, which provides a flexible cushioned surface. Proteoglycans are composed of numerous glycosaminoglycans (GAGs) attached to a core protein. This creates a bottle-brush structure (figure 2) due to the strong negatively charged chains which repel each other.
Collagen provides tensile strength and structural support; and proteoglycans provide compressive strength. The hydrophilic nature of this structure allows it to attract water, which provides a flexible cushioned surface. Proteoglycans are composed of numerous glycosaminoglycans (GAGs) attached to a core protein. This creates a bottle-brush structure (figure 2) due to the strong negatively charged chains which repel each other.
The predominant GAGs in cartilage are chondroitin-4-sulphate, chondroitin-6- sulphate, and keratan sulphate. In the matrix, numerous proteoglycan monomers are attached end-on-end to a molecule of hyaluronan forming a proteoglycan aggregate, which is woven with collagen to form an elastic and compressible structure. Articular cartilage has no intrinsic blood supply and all chondrocyte metabolites and nutrients must diffuse through the matrix to and from the joint space or the vasculature of the subchondral bone. Since the molecular structure of the matrix determines both the rate of diffusion as well as the nature of diffusible particles, normal matrix is a prerequisite for chondrocyte survival and proper functioning.
Degenerative Joint Disease (DJD), or Osteoarthritis (OA), is characterised by articular cartilage destruction, sub-chondral sclerosis, osteophyte production with secondary synovitis, joint effusion and fibrosis of the capsule.
The importance of the matrix is evident in the pathogenesis of OA/DJD, which results when the cartilage matrix fails.[1, 2] Where OA/DJD develops as a result of a predisposing condition (such as trauma, nutritional imbalance, genetic and developmental factors), this is known as 'secondary OA'. When OA appears insidiously, apparently as part of the aging process and without any obvious initiating cause, this is known as 'primary OA'. Changes in the articular cartilage seen in OA/DJD are the results of a complex interplay between the macromolecules of the matrix (GAGs, proteoglycans and collagen) and the chondrocytes that produce the matrix and which are in turn supported by the matrix. At the anatomical level there is initially a softening (chondromalacia) of cartilage, a loss of elasticity, and roughening of the surface. There is progressive loss of cartilage with fibrillation (cleft formation), cracking, and erosion. The underlying bone becomes sclerotic from mechanical pressure, or from a reaction to leakage of synovial fluid as a result of fibrillation. The joint capsule thickens and the synovial surface develops a granular appearance with changes to the synovial fluid (loss of viscosity and changes to the cellular composition). Early on there is osteophyte development at the articular margins. At the biochemical level, the fine balance between production and degradation of cartilage is lost, with the result that there is an overall loss of cartilage. It appears that chondrocytes release a cascade of cytokines, leukotrienes and prostaglandin derivatives triggering an inflammatory response. In addition, these cytokines induce the release of lytic enzymes, including metallo-proteinases, which degrade collagen II and proteglycans. Simultaneously, normal matrix synthesis is inhibited.[3] These events result in a reduced amount and size of GAGs in the matrix, decreased binding between GAGs and collagen and an increase in the amount of water in the matrix. These biochemical changes in the matrix decrease its tensile strength and resilience, and prevent it from functioning normally in transmitting forces, supporting chondrocytes and protecting the subchondral bone. Thus, further injury to chondrocytes occurs and a vicious circle ensues. The disease progresses inexorably with sloughing of cartilage, proliferation and microfractures of subchondral bone, formation of bone cysts and osteophytes, fibrosis of the joint capsule, joint effusion with deterioration of lubrication (loss of viscosity) and nutrient carrying capacity, and the production of 'joint mice'. The clinical result is pain, swelling, and a loss of mobility.
Problems associated with currently available therapies, along with the expanding knowledge of cartilage biochemistry and OA/DJD pathogenesis, have focused research on slowing down degenerative changes and promoting normalisation of cartilage and synovial fluid. [4] This research has identified substances termed chondroprotective agents (CPAs) which appear to address both needs.[5] CPAs have the following attributes:
A combination of CPAs will provide essential nutrients and cofactors for matrix and joint fluid production and anti-oxidants will reduce inflammation and prevent free radical damage.
Examples of compounds that exhibit some of these characteristics are the endogenous molecules of articular cartilage, including hyaluronic acid, glucosamine and chondroitin sulphate.
Hyaluronic Acid (HA) is an unsulphated GAG, which serves as the backbone of proteoglycan aggregates, and as a lubricant and a shock absorber in synovial fluid. HA is not well absorbed orally but has been widely used as an intra-articular treatment of OA in animals.[7]
Glucosamine is an amino-monosaccharide that functions as the precursor of the disaccharide unit in GAGs. There are no food sources of glucosamine. Normally chondrocytes synthesise glucosamine from glucose and an amine (nitrogen and two molecules of hydrogen). Supplying exogenous glucosamine provides the body with additional raw materials for matrix production. Glucosamine also promotes the incorporation of sulphur into cartilage.[9] Numerous in vitro studies have demonstrated that glucosamine stimulates synthesis of proteoglycans and collagen by chondrocytes.[8] Numerous detailed studies on the absorption, distribution and elimination of orally administered glucosamine show an absorption rate as high as 98%.[10]
Chondroitin sulphate is the most abundant GAG in articular cartilage. It is composed of repeating disaccharide units of glucuronic acid and galactosamine sulphate and plays an important structural role in articular cartilage as well as inhibiting many of the degradative enzymes that break down cartilage and synovial fluid.[11,12] While no single compound has yet been found that meets all the defining characteristics of a CPA, by combining nutrients, all criteria can be met.[13]
Sources of Chondroitins - See Chondroprotective Agents (above) for general information.
It appears there are other factors in these extracts, apart from the chondroitins, that have a beneficial effect. It is likely the combination of nutrients found in these whole extracts have a synergistic effect on joint function and help maintain the integrity of cartilage and the underlying bone. An additional supply of collagen provides a rich source of one of the main components of cartilage.
COFACTORS FOR CARTILAGE AND BONE SYNTHESIS
- Support chondrocyte synthesis of collagen and proteoglycans in matrix
- Support production of hyaluronans in joint fluid
- Inhibit cartilage degradation enzymes
- Prevent fibrin formation in synovial fluid and plaque formation in subchondral vessels.[6]
A combination of CPAs will provide essential nutrients and cofactors for matrix and joint fluid production and anti-oxidants will reduce inflammation and prevent free radical damage.
Examples of compounds that exhibit some of these characteristics are the endogenous molecules of articular cartilage, including hyaluronic acid, glucosamine and chondroitin sulphate.
Hyaluronic Acid (HA) is an unsulphated GAG, which serves as the backbone of proteoglycan aggregates, and as a lubricant and a shock absorber in synovial fluid. HA is not well absorbed orally but has been widely used as an intra-articular treatment of OA in animals.[7]
Glucosamine is an amino-monosaccharide that functions as the precursor of the disaccharide unit in GAGs. There are no food sources of glucosamine. Normally chondrocytes synthesise glucosamine from glucose and an amine (nitrogen and two molecules of hydrogen). Supplying exogenous glucosamine provides the body with additional raw materials for matrix production. Glucosamine also promotes the incorporation of sulphur into cartilage.[9] Numerous in vitro studies have demonstrated that glucosamine stimulates synthesis of proteoglycans and collagen by chondrocytes.[8] Numerous detailed studies on the absorption, distribution and elimination of orally administered glucosamine show an absorption rate as high as 98%.[10]
Chondroitin sulphate is the most abundant GAG in articular cartilage. It is composed of repeating disaccharide units of glucuronic acid and galactosamine sulphate and plays an important structural role in articular cartilage as well as inhibiting many of the degradative enzymes that break down cartilage and synovial fluid.[11,12] While no single compound has yet been found that meets all the defining characteristics of a CPA, by combining nutrients, all criteria can be met.[13]
Sources of Chondroitins - See Chondroprotective Agents (above) for general information.
- Glucosamine hydrochloride is the more concentrated form of glucosamine providing 83% of actual glucosamine compared with 63% for glucosamine sulphate
- Bovine tracheal cartilage: A freeze dried powder <150 micron particle size contains 20-28% GAGS as chondroitin-4-sulphate and chondroitin-6-sulphate. In this unrefined form it also provides 23% collagen (mostly type II) and calcium.
- Shark cartilage: A freeze dried powder <150 micron particle size contains 7-12% GAGS, mainly chondroitin-6-sulphate, 20% collagen (type I&II), and 50% calcium hydroxyapatite. 15% of the total GAGs is keratan sulphate. The anti-angiogenic effect of shark cartilage may prevent the vascularisation and degradation of cartilage and block the progression of OA.[16] Dogs with severe secondary OA responded to oral supplementation with shark cartilage extract and regressed following cessation of treatment.[17]
- Green lipped mussel - A freeze dried powder from Marlborough Sounds mussels, contains a unique combination of complex proteins, GAGs (2-3%), amino acids, fatty acids, and naturally occurring chelated minerals. It has a strong action thought due to a heat sensitive glycogen fraction.[18] More recently there has been a focus on the fatty acid components.[19]
It appears there are other factors in these extracts, apart from the chondroitins, that have a beneficial effect. It is likely the combination of nutrients found in these whole extracts have a synergistic effect on joint function and help maintain the integrity of cartilage and the underlying bone. An additional supply of collagen provides a rich source of one of the main components of cartilage.
COFACTORS FOR CARTILAGE AND BONE SYNTHESIS
- Vitamin C is required for the development of connective tissues such as muscle, cartilage and bone. The synthesis of GAGs associated with collagen fibres increases 30-90%, and the deposition into the extra-cellular matrix increases 80% in the presence of ascorbic acid.[21]
- Kelp Air dried kelp powder provides a natural and wide range of balanced chelated minerals essential to cartilage and bone development.
- Vitamin E, provided in its natural form, is a potent anti-oxidant and prostaglandin inhibitor. It prevents lipid peroxidation and protects nutrients from oxidation. It appears to inhibit the breakdown of cartilage and stimulates new cartilage generation.[22]
- Boron Epidemiological studies have shown an inverse relationship between soil boron levels and the prevalence of osteoarthritis.[23]
- Niacinamide (Vitamin B3) has been shown to improve joint function.[24]
- Magnesium is essential to the function of more than 300 enzymes and studies have shown that more than half the population (human) may be deficient. Up to 50% of all magnesium is found in bone. Magnesium supplementation has a suppressive effect on bone turnover rate. Inflammatory activity around joints is influenced by the health of bone and cartilage and magnesium plays a significant role in this process.[25]
- Manganese is essential for the biosynthesis of GAGs and proteoglycans, and is required for bone mineralisation and the synthesis of connective tissue in cartilage and bone.[26]
- Zinc is the second most important trace element and is provided as an amino acid chelate to enhance its utilisation. About 85% of zinc reserves are found in bone and muscle. Serum zinc levels may be depressed in arthritic patients.[27,28]
- Copper is important in the mineralisation of bone and is a component of many enzymes that catalyse reactions involving oxygen or related radicals (free radical scavenging). If zinc is supplemented it should be balanced with copper in roughly a 10:1 ratio.[29]
- Selenium Following treatment with selenium and vitamin E, a correlation was found between pain relief and increased blood levels of glutathione peroxidase.[30]
- MSM (methylsulphonylmethane) present naturally in milk, fruit and vegetables, is a dietary source of sulphur which is essential to the formation of connective tissue.[31,32]
- Devils Claw is a herb widely used to support joint function.
- Yeast a rich source of amino acids and B vitamins.
REFERENCES
1. Brandt K, Radin E. The physiology of articular stress: Osteoarthritis. Hosp Pract. 1987;1:103-126
2. Martin DF. Pathomechanics of knee osteoarthritis. Med Sci Sports Exerc. 1994;26(12):1429-1434
3. Rosenberg AE. Joints. In: Cotran RS, Kumar V, Robbins S, eds. Pathologic Basis of Disease. Philadelphia, Pa: WB Saunders; 1994:1243-1248
4. Serni U. Profile of glucosamine as an example of a slow-acting drug in osteoarthritis. In: Proceedings of the 18th Congress of Rheumatology. Rev Esp Rheumatolog. 1993;20(suppl):222|
5. Bassleer C, Henrotin Y, Franchiment P. In Vitro evaluation of drugs proposed as chondroprotective agents. Int J Tissue React. 1992; 14:231-241
6. Ghosh P. Second-line agents in osteoarthritis. In: Second-line Agents in the Treat-ment of Rheumatic Diseases. New York, NY: Marcel Dekker; 1992:363-427
7. McIllwraith CW. Diseases of joints, tendons, ligaments and related structures. In: Stashak TS, ed. Adams Lameness in Horses. Philadelphia, PA; Lea & Feibiger; 1984:368-369
8. Jimenez SA. The effects of glucosamine on human chondrocyte gene expression. Madrid, Spain. The Ninth Eular Symposium; 1986:8-10
9. Raiss R. Einfluss von D-glucosaminsulfat auf experimentell geschaedigten gelenkknorpel. Fortschr Med. 1985; 103(24):658-668
10. Setnikar I et al. Pharmacokinetics of glucosamine in the dog and man. Arzneim Forsch 36(4), 729-735, 1986
11. Bassleer C, Malaise M Chondroitin sulphate: Its in vitro effects on human articular chondrocytes cultivated in clusters. Singapore: The Third International Congress of the Osteoarthritis Research Society; 1997
12. Paroli E et al. A pharmacological approach to glycosaminoglycans. Drugs Exp Clin Res. 1991;17(1):9-20.
13. Bucci L. Chondroprotective agents: Glucosamine salts and chondroitin sulfates. Townsend Letter for Doctors. 1994;1:52-54
14. Arthritis and Rheumatism, Vol 42, No. 9 (supplement) #1111 Sept, 1999
15. Prudden JF, Balassa LL. The biological activity of Bovine Cartilage preparations. Seminars Arth. and Rheum. 3:278-321, 1974.
16. Neovascularisation and its role in the osteoarthritic process. Brown.R, Weiss.J, Ann Rheum Dis, Vol. 47(11):881-5,1988.)
17. Lane IW. Shark Cartilage: Its potential medical applications. J Advance Med. 4(4):26, 1991.
18. Miller TE, Dodd J, Ormrod DJ, Geddes R. Anti-inflammatory activity ofglycogen extracted from Perna Canaliculus (NZ green lipped mussel). Agents Actions 38, Special Conference Issue (1993)
19. Geusens P, Wouters C, Nijs J, Jiang Y, Dequeker J. Long term effects of omega-3 fatty acid supplementation in active Rheumatoid Arthritis. A 12 month, double-blind, controlled study. Arthritis Rheum.37(6):824-9.1994.
20. Animal Health Services Centre, Massey University, Palmerston North. Final report on the Efficacy of Green Lipped Mussel Extract in the Management of Degenerative Joint Disease in Dogs. September 1999. Study No. AHSC-75353
21. Kao et al. Exper Mol Pathol 53:1, 990.
22. Schwartz,E.R. The modulation of osteoarthritic development by vitamin C and E. Int J Vit Nutr Res 26S:141-146, 1984.
23. de fabio A. Treatment and prevention of Osteoarthritis. Townsend Letter for Doctors, Feb-Mar, 1990:143-148.
24. Kaufman W. Am J Geriatr Soc 3:927, 1955.
25. Dimai,H.Pet al, Journal of Clinical Endocrinology and Metabolism:83(8): 2742-8, 1998
26. The need for manganese in bone development by the rat. Proc Soc Exp Biol Med:59,254-5,1945
27. Grennan DM et al. Serum Copper and Zinc Levels in Rheumatoid arthritis and Osteoarthritis. NZ Med J 91(652):47-50, 1980.
28. Naveh, Yehezkel, et al. Plasma and Urinary Zinc and relationship to disease activity, Journal of Rheumatol:24(4),634-6, 1997)
29. Effects of Copper Supplementation on Ceruloplasmin and Copper-Zinc Superoxide Dismutase in Arthritic Patients, Disilvestro, R.A., et al, Journal of the American College of Nutrition: 11(2),177-180,1992.
30. Jameson S et al. Pain relief and selenium balance in patients with connective tissue disease and osteoarthritis: A double blind selenium tocopherol supplementation study. Nutr Res Suppl: 391-7, 1985
31. IuV Marav'ev, MS Venikova, GN, Plesovkskaia, TA Riazantseva and IaA Sigidin, 'Effect of dimethyl sulfoxide and dimethyl sulfone on a destructive process in the joints of mice with spontaneous arthritis'. Patol Fiziol Eksp Ter, (2) (Mar/Apr 1991): 37-9.
32. Robert J. Herschler 'Use of methylsulfonylmethane to relieve pain and nocturnal cramps to reduce stress-indiced deaths in animals' US Patent 4973605, issued Nov. 27, 1990
33. Butenko et al 1993. Anti-inflammatory properties and inhibition of leukotriene C4 biosynthesis in vitro by flavanoid baicalein from Scutellaria baicalensis georgy roots. Agents Actions 39 Spec No. C49-51, 1993
34. Steinegger and Hovel, 1972. Analytische und bioligische Untersuchungen an Salicaceen-Wirkstoffen, insbesondere an Salicin. 1 und 2 Mittg Pharm Acta Helv, 47, 133, 222, 1972.
1. Brandt K, Radin E. The physiology of articular stress: Osteoarthritis. Hosp Pract. 1987;1:103-126
2. Martin DF. Pathomechanics of knee osteoarthritis. Med Sci Sports Exerc. 1994;26(12):1429-1434
3. Rosenberg AE. Joints. In: Cotran RS, Kumar V, Robbins S, eds. Pathologic Basis of Disease. Philadelphia, Pa: WB Saunders; 1994:1243-1248
4. Serni U. Profile of glucosamine as an example of a slow-acting drug in osteoarthritis. In: Proceedings of the 18th Congress of Rheumatology. Rev Esp Rheumatolog. 1993;20(suppl):222|
5. Bassleer C, Henrotin Y, Franchiment P. In Vitro evaluation of drugs proposed as chondroprotective agents. Int J Tissue React. 1992; 14:231-241
6. Ghosh P. Second-line agents in osteoarthritis. In: Second-line Agents in the Treat-ment of Rheumatic Diseases. New York, NY: Marcel Dekker; 1992:363-427
7. McIllwraith CW. Diseases of joints, tendons, ligaments and related structures. In: Stashak TS, ed. Adams Lameness in Horses. Philadelphia, PA; Lea & Feibiger; 1984:368-369
8. Jimenez SA. The effects of glucosamine on human chondrocyte gene expression. Madrid, Spain. The Ninth Eular Symposium; 1986:8-10
9. Raiss R. Einfluss von D-glucosaminsulfat auf experimentell geschaedigten gelenkknorpel. Fortschr Med. 1985; 103(24):658-668
10. Setnikar I et al. Pharmacokinetics of glucosamine in the dog and man. Arzneim Forsch 36(4), 729-735, 1986
11. Bassleer C, Malaise M Chondroitin sulphate: Its in vitro effects on human articular chondrocytes cultivated in clusters. Singapore: The Third International Congress of the Osteoarthritis Research Society; 1997
12. Paroli E et al. A pharmacological approach to glycosaminoglycans. Drugs Exp Clin Res. 1991;17(1):9-20.
13. Bucci L. Chondroprotective agents: Glucosamine salts and chondroitin sulfates. Townsend Letter for Doctors. 1994;1:52-54
14. Arthritis and Rheumatism, Vol 42, No. 9 (supplement) #1111 Sept, 1999
15. Prudden JF, Balassa LL. The biological activity of Bovine Cartilage preparations. Seminars Arth. and Rheum. 3:278-321, 1974.
16. Neovascularisation and its role in the osteoarthritic process. Brown.R, Weiss.J, Ann Rheum Dis, Vol. 47(11):881-5,1988.)
17. Lane IW. Shark Cartilage: Its potential medical applications. J Advance Med. 4(4):26, 1991.
18. Miller TE, Dodd J, Ormrod DJ, Geddes R. Anti-inflammatory activity ofglycogen extracted from Perna Canaliculus (NZ green lipped mussel). Agents Actions 38, Special Conference Issue (1993)
19. Geusens P, Wouters C, Nijs J, Jiang Y, Dequeker J. Long term effects of omega-3 fatty acid supplementation in active Rheumatoid Arthritis. A 12 month, double-blind, controlled study. Arthritis Rheum.37(6):824-9.1994.
20. Animal Health Services Centre, Massey University, Palmerston North. Final report on the Efficacy of Green Lipped Mussel Extract in the Management of Degenerative Joint Disease in Dogs. September 1999. Study No. AHSC-75353
21. Kao et al. Exper Mol Pathol 53:1, 990.
22. Schwartz,E.R. The modulation of osteoarthritic development by vitamin C and E. Int J Vit Nutr Res 26S:141-146, 1984.
23. de fabio A. Treatment and prevention of Osteoarthritis. Townsend Letter for Doctors, Feb-Mar, 1990:143-148.
24. Kaufman W. Am J Geriatr Soc 3:927, 1955.
25. Dimai,H.Pet al, Journal of Clinical Endocrinology and Metabolism:83(8): 2742-8, 1998
26. The need for manganese in bone development by the rat. Proc Soc Exp Biol Med:59,254-5,1945
27. Grennan DM et al. Serum Copper and Zinc Levels in Rheumatoid arthritis and Osteoarthritis. NZ Med J 91(652):47-50, 1980.
28. Naveh, Yehezkel, et al. Plasma and Urinary Zinc and relationship to disease activity, Journal of Rheumatol:24(4),634-6, 1997)
29. Effects of Copper Supplementation on Ceruloplasmin and Copper-Zinc Superoxide Dismutase in Arthritic Patients, Disilvestro, R.A., et al, Journal of the American College of Nutrition: 11(2),177-180,1992.
30. Jameson S et al. Pain relief and selenium balance in patients with connective tissue disease and osteoarthritis: A double blind selenium tocopherol supplementation study. Nutr Res Suppl: 391-7, 1985
31. IuV Marav'ev, MS Venikova, GN, Plesovkskaia, TA Riazantseva and IaA Sigidin, 'Effect of dimethyl sulfoxide and dimethyl sulfone on a destructive process in the joints of mice with spontaneous arthritis'. Patol Fiziol Eksp Ter, (2) (Mar/Apr 1991): 37-9.
32. Robert J. Herschler 'Use of methylsulfonylmethane to relieve pain and nocturnal cramps to reduce stress-indiced deaths in animals' US Patent 4973605, issued Nov. 27, 1990
33. Butenko et al 1993. Anti-inflammatory properties and inhibition of leukotriene C4 biosynthesis in vitro by flavanoid baicalein from Scutellaria baicalensis georgy roots. Agents Actions 39 Spec No. C49-51, 1993
34. Steinegger and Hovel, 1972. Analytische und bioligische Untersuchungen an Salicaceen-Wirkstoffen, insbesondere an Salicin. 1 und 2 Mittg Pharm Acta Helv, 47, 133, 222, 1972.